The BEAD Study
Does the baby head elevation cushion (Fetal Pillow®) make an emergency caesarean section, when the cervix is fully dilated, safer for māmā and pēpi?
BEAD study video – English
BEAD study video – te reo Māori
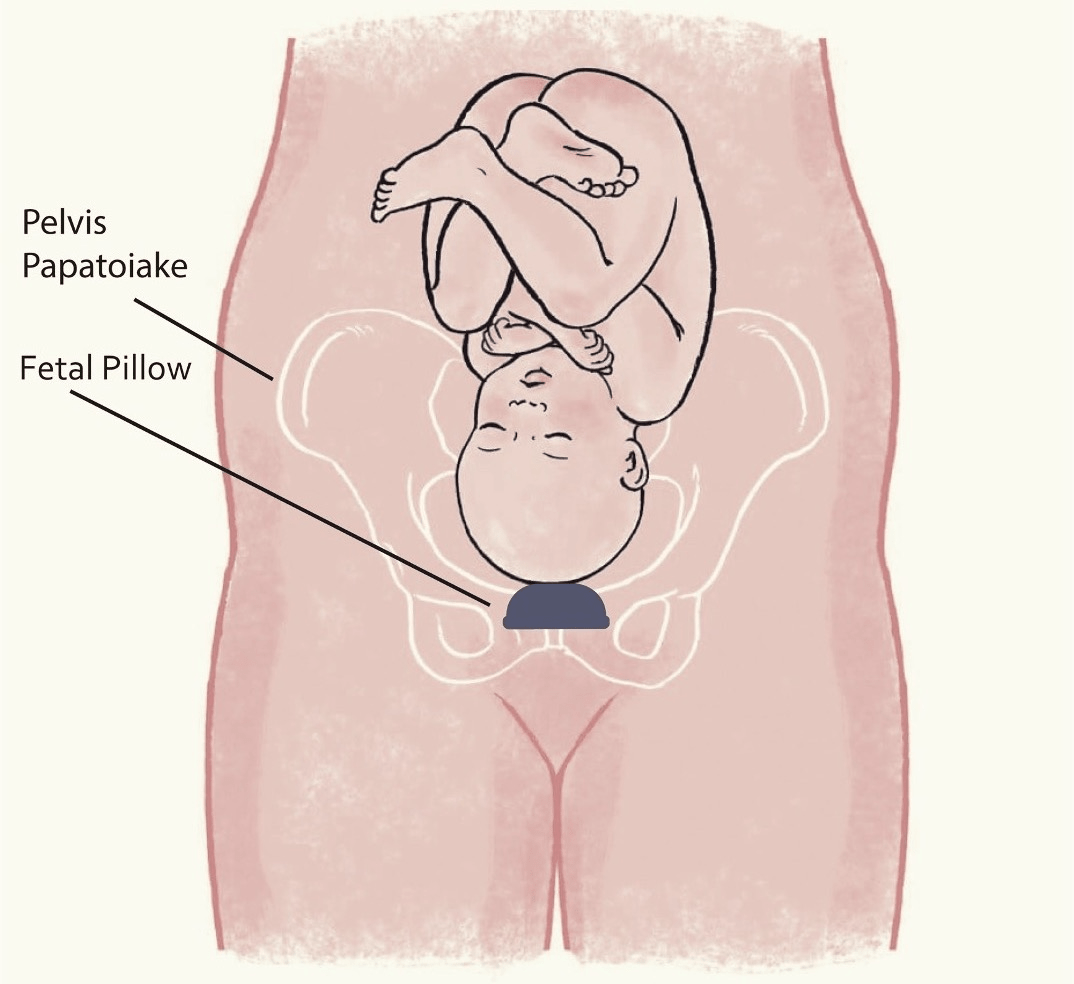
What is a Fetal Pillow®?
The Fetal Pillow® is a small, soft silicone balloon that is placed in the vagina before the caesarean and many doctors have started to use it. The balloon aims to elevate the baby’s head to make the caesarean section easier.
What is the BEAD Study?
We are carrying out the BEAD Study to find out whether using a Fetal Pillow® for those women needing a caesarean section at full dilatation reduces the risk of injuries to the mother and baby. The study will include 400 patients.
The purpose of this study is to find out whether using the Fetal Pillow® reduces complications for mothers and babies who undergo a fully dilated caesarean section (primarily measured by extensions of the maternal uterine incision), and whether its use is cost effective.
Why is the BEAD Study important?
In Aotearoa New Zealand around 1500 birthing people (2-3% of all births) have an emergency caesarean section at full dilatation (10cm) each year. Caesarean section at this stage of labour is associated with increased risks such as injury to the uterus, resulting in longer operating time and increased blood loss. This may be due to difficulty with delivering the baby’s head which can be deep in the pelvis. We also know that having a caesarean section at full dilatation increases the risk of having a preterm birth in a future pregnancy.
We want to know whether using the Fetal Pillow® safely reduces these risks.
Who can take part in the BEAD Study?
The BEAD Study is happening at four hospitals in Auckland, New Zealand (Auckland City, Waitakere, North Shore, and Counties Manukau).
You can take part in this study if you are pregnant with one baby that is head down (cephalic) and if you are more than 36 weeks pregnant when you are in labour, and need a caesarean section when you are 10cm dilated.
This study may not be suitable if your baby is unwell, you are having twins or triplets, are less than 37 weeks’ pregnant or have a baby that is not head down (e.g. breech).
We don’t know who needs an emergency caesarean section at full dilatation before labour so if you become eligible you may be asked if you would like to take part in labour.
Please let your midwife or obstetrician know if you would like to take part or contact the BEAD study team to let them know you are interested.
What is involved in the BEAD Study?
Participation in this study is voluntary. If you become eligible you will be approached in labour and asked if you would like to take part.
If you agree to take part, you will have the Fetal Pillow® placed in the vagina (by your surgeon) immediately prior to your emergency caesarean section at full dilatation. This will either be inflated (with water) or not inflated. The anaesthetist (who is in charge of making you comfortable during the operation) will be responsible for the Fetal Pillow® being inflated or not inflated. You, your midwife and your surgeon won’t know if the balloon has been inflated and you can’t choose which group you are in.
After your caesarean, a member of our research team will visit you while you are in hospital and ask your consent to collect information about you and your baby.
We know that complications of caesarean are linked with preterm birth. As a sub-study, we are interested in seeing if you have a preterm birth in a future pregnancy, and will also ask if you consent to our research team finding out if you have another birth in the next 5 years and whether this next birth is preterm (<37 weeks).
You and your baby will not need to have any tests for this study after your birth.
Are there any risks?
The Fetal Pillow® is already used in many maternity hospitals across New Zealand. Previous international studies have not shown any harms to mothers or babies from its use. This study is designed to assess the benefits (and risks) of using the Fetal Pillow® with the aim to improve maternity care in the future.
Who is organising the study?
The BEAD Study is led by:

Jordon Wimsett

Robin Cronin

John Thompson

Katy Antony

Lynn Sadler

Charlotte Oyston
Matthew Drake

Evon Huang & Maria Boston

Meghan Hill

Karaponi Okesene-Gafa

Erena Browne

Jane Alsweiler
Who is funding the study?
The BEAD Study is funded by grants from the Health Research Council of New Zealand, the Auckland Medical Research Foundation (AMRF), the Mercia Barnes Trust of RANZCOG, and the Auckland Hospital Research and Endowment Fund.
Participant experiences from the BEAD Feasibility Study
Prior to the BEAD Study we undertook a one year feasibility study at two sites in New Zealand. The feasibility study had the same inclusion criteria and trial processes, and we asked those approached about the study in labour to share their experience in a follow-up survey after birth. Overall, 70% of those approached in labour consented to the randomised trial.
Find out more
Contact the BEAD Clinical Research Coordinators via email at: thebeadstudy@auckland.ac.nz
- Auckland City Hospital, Dr. Lynn Sadler
- Middlemore Hospital, Dr. Charlotte Oyston
- North Shore and Waitakere Hospitals, Dr. Kathleen Antony